
On a standard chest radiography and CT scan, the enlargement of the aortic arch and the tortuous dilated aorta can be observed. 6) The diagnostic work-up includes radiologic, endoscopic, and manometric studies. The association of suggestive symptoms, such as progressive intolerance to solids with concomitant weight loss along with the results of imaging and other diagnostic studies provide a high index of suspicion. There is no gold standard diagnostic procedure for dysphagia aortica. 3) As a result, the esophagus is pushed and compressed by the aorta against the cardiac chambers, which are anterior in location. 4) The aging process and the accompanying degenerative changes with the loss of elasticity causes a dilated, elongated, and distorted aorta, which may result in a so-called reverse C- or reverse S-shaped aorta. Then, the esophagus lies on the left side of the aorta and penetrates the diaphragm through the diaphragmatic histus. This area is called the aortoesophageal decussation site. The esophagus crosses the aorta anteriorly in the lower third of the posterior mediastinum. The esophagus normally begins on the right side of the thoracic aorta and then descends. Although we recommended surgical correction of the aortic aneurysm or percutaneous endoscopic gastrostomy, the patient declined any invasive procedures and she was transferred to a nursing home on the 12 th hospital day. We concluded that the symptoms and the results of the imaging studies were consistent with dysphagia aortica. The upper gastrointestinal barium study revealed marked extrinsic compression of the distal esophagus just above the esophagogastric junction ( Fig.
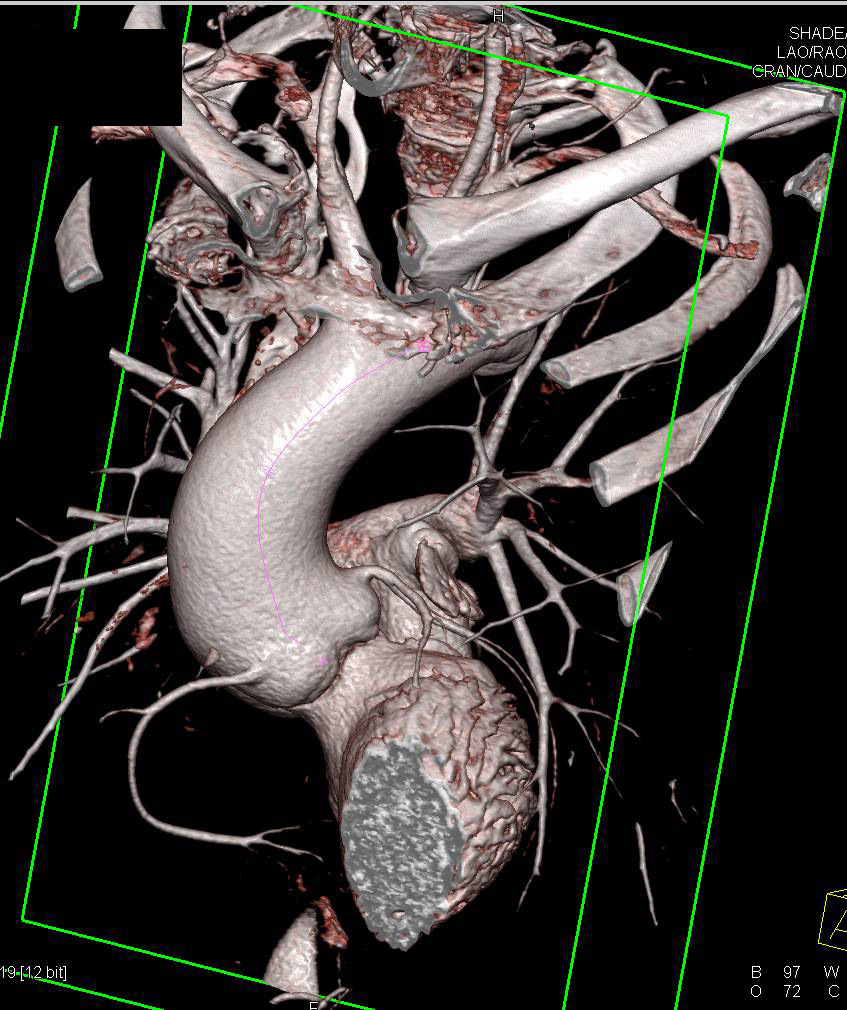
There was an intramural thrombus at the level of the descending aorta. The diameters of the ascending thoracic aorta, the descending thoracic aorta, and the proximal abdominal aorta were 7 cm, 6 cm, and 5.3 cm, respectively. Computed tomography (CT) of the chest demonstrated an enlarged, tortuous aorta ( Fig. Her chest radiograph revealed blunting signs at both costophrenic angles, cardiomegaly with a cardio-thoracic ratio of 0.8, and an enlarged, tortuous aorta ( Fig. An electrocardiogram (ECG) demonstrated a left axis deviation and left ventricular hypertrophy. The laboratory findings were as follows: the white blood cell (WBC) count was 5,100/mm 3, the hemoglobin was 11.8 g/dL, the platelet count was 151,000/mm 3, the blood urea nitrogen (BUN) was 39.8 mg/dL, the creatinine was 1.5 mg/dL, the total protein was 5.8 g/dL, the albumin was 3.2 g/dL, the lactate dehydrogenase (LDH) was 546 IU/L, and the creatinine phosphokinase (CPK) was 380 IU/L. The physical examination showed a diastolic murmur at the right upper sternal border and a pansystolic ejection murmur at the left lower sternal border. The blood pressure was 130/90 mmHg, the pulse rate was 64 beats/min, the respiratory rate was 28 breath/min, the body temperature was 36.0℃, the height was 1.43 m, and the body weight was 37 kg. On admission to the hospital, she had a chronically ill appearance. She had undergone vertebroplasty due to multiple compression fractures of the thoracic and lumbar vertebrae 5 years ago.
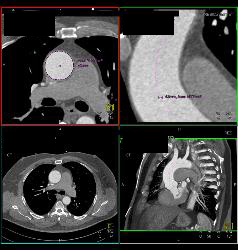
Because of old age, she did not undergo an operation and she was on symptomatic treatment. The medical history revealed that she had been diagnosed with primary hypertension, an ascending aortic aneurysm, congestive heart failure, moderate aortic regurgitation, and moderate mitral regurgitation 6 years previously. Three days before seeking evaluation at our hospital, she had difficulty in swallowing liquids, along with nausea and vomiting. Because of her progressive dysphagia to solids for the last 6 months, she had ingested only semisolids and liquids. An 86-year-old woman presented with worsening nausea and vomiting.


 0 kommentar(er)
0 kommentar(er)
